
How can procurement help project teams manage and invest their budgets wisely while fostering innovation and delivering breakthrough savings?
Editors note: this is the second in a three-part series by Mark Davis, exploring the opportunities for procurement leaders to evolve into innovation enablers (Read Part 1 – Procurement Enabled Innovation – A Case Study from the Cell Therapy World). While the series shares examples from the pharmaceutical industry, the actions and learnings can be applied across sectors. PI
BACKGROUND
In today’s pharmaceutical sector, speed is the name of the game. Pharmaceutical companies rely on the sanctity of patents to protect their intellectual property. A new drug has a market advantage due to a company’s right to exclude others from utilizing the underlying intellectual property for often a period of 20-years. Pharma’s pricing power traditionally derives from this exclusivity.
The pharma sector is changing considerably. There is growing pressure from customers, including insurance companies and governments, to aggressively control their costs in order to treat a growing population with increasingly limited resources. This increased pricing pressure only magnifies the need-for-speed pressure on the development scientists responsible for figuring out how to make new innovative drugs.
The game has evolved from patent derived barriers to entry, to now include harnessing first-to-market advantages. A second place competitor drug is at significant disadvantage as it not only must be differentiated through performance, but also the first-mover’s sales team has a head-start in securing market share. As a result, the scientific teams responsible for developing a new drugs place higher priority on quality and speed to quickly identify a manufacturing process that works, but not always efficient.
THE CHALLENGE
In this case study, the project team needed speed, quality, and cost reductions and were willing to accept higher risk to deliver it.
Common procurement practice would be to analyze the supply chain, direct suppliers (and suppliers of suppliers) to identify opportunities to drive competition or consolidate spend to gain economies of scale. I did both, without affecting the manufacturing process. However, competition and consolidation can only deliver so much cost impact. The rock-bottom price of producing a drug is driven by the underlying economics such as: raw material cost, number of steps in the production process, efficiency, throughput, etc. As such, cost can be lowered only so much by traditional procurement levers.
Making most drugs is like many other sequential manufacturing processes. To create the product requires multiple steps performed in sequence with each step producing a more complex and value added product. In a chemical drug process, there are losses associated with each step, and the more steps in the chain the higher the impact these cumulative losses have on the final cost of the drug.
When developing a drug, the chemists evaluate different options for making a drug, starting with identifying the simplest process with the least amount of manufacturing steps, while eliminating as much costly, dangerous, or environmentally hazardous materials as possible.
However, with a finite amount of chemists on a team and priorities being speed and product quality, the effort is focused on finding a process that works well enough to make the final drug. Procurement’s traditional contributions are largely commercial in nature.

Balancing Cost vs. Speed
How can procurement deliver extraordinary cost reduction for a drug when the project team’s priority is not cost, but is instead speed?
SETTING THE SCENE
The story starts during a lunch meeting with my Project Manager and her Program Manager. My Project Manager and I had aligned a strategy and game-plan before the meeting. We had recently been asked to provide a budget for over a metric ton of developmental drug for a high-priority cancer drug. A rather large multi-million dollar budget had been proposed to meet demand.
The Program Manager shared that he would have a hard time securing the budget for the next campaign. On top of the focus on keeping project timelines, the team now also needed to reduce the cost to avoid having to reduce funding to other programs. What could procurement do to help?
I proposed to my customers that we invest! We identified savings from the existing supply chain and knew we could over-spend our already approved budgets by as much as 10% without incurring too many complaints from finance. This freed up roughly several hundred-thousand dollars for investment. I proposed that we develop a population of 2nd-tier suppliers to drive competition to supply raw materials. However, more aggressively, I proposed to invest heavily in external process R&D support focused solely on cost reduction efforts at-risk!
THE SOLUTION – Go to China Young Man!
I needed more scientists. My plan was to rent some!
I had already received detailed process descriptions from the scientists to support previous outsourcing efforts, from which I prepared a detailed cost map. With help from the internal team, we identified the biggest cost drivers and outlined an experimental plan to attack them.
With the funding available, and the team behind me, we took a risk and secured access to additional scientific resources from a Chinese CDMO effectively tripling the scientific output! We took that new intellectual capacity and set them loose to try and solve the biggest process cost drivers.
In essence, we amplified the productivity of our internal scientists and paid for these resources through funds freed from previous and current campaigns.
Our Chinese partners eliminated expensive materials, increased the efficiency of targeted process steps, and were able to produce higher throughputs from each process step. After a roughly 2-month period, we had enough data from the experiments to pull everything together into a new process.
However, once again, time and money were our enemies. We had run out of both and still needed to prove all of the new modifications could come together in a viable new large-scale process. By chance, the next production campaign afforded an opportunity to deliver on the investment.
DEUTSCHLAND ZUR RETTUNG!!
The program team needed another production campaign and had approved a new, albeit reduced, budget. We selected a German custom manufacturer (CMO) for our next campaign. I had a collaborative and trusting relationship with the supplier’s business development manager and shared what we had been working on.
My counterpart was quite intrigued; his own technical team had expressed concern at the high volumes of materials (and cash) they would need to purchase in advance to deliver the campaign. One material would even need to be shipped via sea freight from China due to the cost prohibitive nature of all faster transportation modes. We decided to pull the two scientific teams together and discuss the results provided by our Chinese collaborator.
As the meeting concluded, the teams were left with two choices:
- Execute the current inefficient process and accept longer project timelines because of the sea-shipment, or
- Utilize the new data and complete the development of the more efficient process – reinvesting the time gained from eliminating the material requiring sea-shipment.
The joint scientific teams believed the data was compelling enough to explore the new process and decided to take the development risk.
Our German partners were the ones who would have to complete the development – addressing quality deficiencies that were identified from the Chinese pilot production.
My external counterpart and I had to figure out how to convince his management to support the consumption of virtually all of the German collaborator’s scientific capacity without exceeding the originally agreed cost of the campaign. If, for illustrative purposes, the agreed upon cost of the original campaign was:
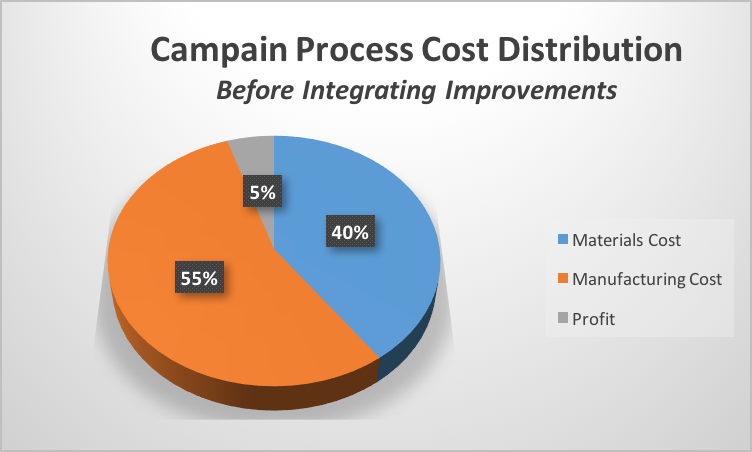
We both knew the new cost of the materials in the new process and the expected increase in throughput’s impact on total product cost . In order to secure the needed scientific capacity, I proposed that if the supplier were to allocate their scientific capacity at no additional up-front cost to me, I would be willing to evenly divide the savings (illustrated below) and actually increase their profit.
It should be noted that the CMO selected was a critical component to the success, being one that also invested in the endeavor and partner on the central aspects in sourcing management:
- First, the CMO was given the chance to evaluate the new process and test in the laboratory to build confidence in the alternative process, allowing for fair judgement that the shared profits have a realistic chance to balance CMO´s investment, if the project succeeded;
- Second, the CMO was supported by our internal scientists when piloting the process through full transparency and utilization of their available scientific capacity when needed and notably investing themselves heavily in transparent and very collaborative external sourcing and project management;
- Third, an agreement covering the CMO’s financial risks – if the new process failed we covered the cost of all of the purchased materials;
- Fourth, the German CMO was financially stable and acted as a partner to our business, taking a long-term support perspective. They were strong business partner taking pride in and being positively recognized by multiple business opportunities from their history of delivering on programs. And, finally the CMO was open to acting as the extended resource and manufacturing partner.
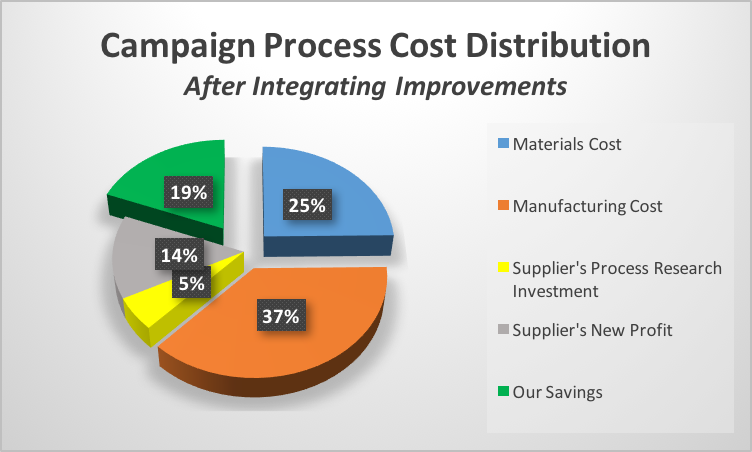
We had a deal!
THE RESULT
After intensive collaboration between the scientific teams over the span of several months, the new process delivered a quality product and resulted in a 38% cost reduction (split with our German partners as agreed) relative to the originally proposed cost of the drug. The savings realized from this campaign represented a 3200% return on investment relative to the original amount spent at-risk with the Chinese process research collaborator and developing the 2nd tier supply chain.
Since it was agreed that the process and associated intellectual property belonged to the us, procurement was able to take the improved process and realize further benefit by placing part of future demand back to a lower-cost Chinese manufacturer appropriate for larger scale production while maintaining redundancy with the German manufacturer. When we later transferred a campaign to China, the cost was reduced by an additional 43% versus the German campaign due to lower labor and fixed-asset costs at the time!
CONCLUSION
This case study is an illustration of how procurement can build bridges leading to breakthrough innovative value.
In this example, procurement was able to affect the underlying technical cost structure of the product by working with the technical team to increase their scientific output, without seriously impacting the project’s higher priority timelines. As a partner to the business, procurement created the opportunity for the project team to deliver speed and quality at a lower cost by taking a strategic risk significantly returning its investment.
Please note: this article does not represent, be it expressed or implied, the views, opinions, policies, strategies, or other, of the Author’s employer. The Author wrote the article in his private capacity.





